論文紹介LUCUBRATIONS
Canine AV nodal artery: anatomical variations and a detailed description of cannulation technique
TAKAO MITSUOKA, AMIR PELLEG, ERIC L. MICHELSON, AND LEONARD S. DREIFUS
Cardiovascular Division, Lankenau Medical Research Center, Philadelphia 19151; and Department of Medicine, Jefferson Medical College of Thomas Jefferson University, Philadelphia, Pennsylvania 19107
TAKAO MITSUOKA, AMIR PELLEG, ERIC L. MICHELSON, AND LEONARD S. DREIFUS Canine AV nodal artery: anatomical variations and a detailed description of cannulation technique. Am. J. Physiol. 253 (Heart Circ. Physiol. 22): H968- H973, 1987 -Cannulation of the atrioventricular (AV) nodal artery for selective perfusion of the AV node is a useful physiological method for evaluating the direct effects of pharmacological agents on the AV node. However, previous reports have not included a detailed description of the technique for AV nodal artery cannulation. Furthermore, successful cannulation is dependent on familiarity with the anatomical variations of the AV nodal artery [i.e., the most superior posterior septal artery (PSA)], which supplies blood to the AV nodal region and the posterior descending artery (PDA). The purpose of this report is to describe in detail the technique for cannulation of the AV nodal artery as well as the common anatomical variations of this artery. The anatomy of the PDA and PSA was studied at postmortem examination with ink injection in 30 dogs. Verification of the anatomical location of the AV nodal artery was aided by the induction of transient AV nodal conduction block following intracoronary administration of acetylcholine in the beating heart, as was done in previous studies. Two main variations and two subtypes of PDA anatomy and three main variations of AV nodal artery were found. Based on the present findings, an improved technique for cannulation of the AV nodal artery was established. Using this technique, we achieved a high rate of successful cannulation.
atrioventricular node
CANNULATION OF THE CANINE atrioventricular (AV) nodal artery, for selective perfusion of the AV node, has been employed as a useful physiological method to evaluate the direct effects of pharmacological agents on the AV node without interference from systemic baroreflexes 1) 2) 3) 4) 7) 8) 12) 15) 16) 17) 18).
This method, originally introduced by Nadeau and Amir-Jahed 11) , has been used and later modified by other investigators 1) 2) 3) 4) 7) 8) 12) 15) 16) 17) 18). However, previously published methods are lacking in two respects: 1) no detailed information was given with regard to the anatomical variations of the AV nodal artery, i.e., the most superior posterior septal artery, and 2) no specific anatomical guidelines for successful cannulation were furnished.
Thus the purpose of this study was to establish a reproducible, reliable technique for successful cannulation of the AV nodal artery to facilitate electrophysiological and electropharmacological studies of the AV node. To do this, the anatomical variations of the posterior descending artery (PDA) and the AV nodal artery were studied in detail, allowing us to define readily identifiable, reliable anatomical landmarks for cannulation of the AV nodal artery.
MATERIALS AND METHODS
Twenty mongrel dogs (25-30kg, either sex) were premedicated with intramuscular morphine sulfate (2mg/kg), anesthetized with intravenous α-chloralose (100mg/kg), intubated with a cuffed endotracheal tube, and ventilated with room air supplemented with O2 as necessary using a Harvard respirator. Three quadripolar electrode catheters were introduced through the left femoral vein, the right jugular vein, and the left carotid artery to record bipolar electrograms from the right atrium, the right ventricle, and the His bundle, respectively. The right atrial catheter was also used for right atrial pacing. Systemic atrial pressure was recorded with a Millar catheter tip electrotransducer introduced via the left femoral artery.
Definitions. The AV nodal artery is the most superior posterior septal artery, with the AV nodal branch being that which perfuses the AV nodal region. When the PSA is a relatively small vessel without any branches in its proximal portion, then PSA is the AV nodal branch (see below).
Identifying the posterior septal artery. A bilateral thoracotomy was performed at the level of the fifth inter- costal space. The animal was then positioned on its left side. The pericardium was incised along the right phrenic nerve. The heart was suspended in a pericardial cradle to enable visualization of the PDA and the crux of the heart (Fig. 1). The latter is the groove between the coronary sinus, at its point of entrance into the right atrium, and the inferior vena cava, where the AV sulcus crosses the posterior sulcus. Although the PDA was not always a large vessel immediately visible, the crux of the heart was a reliable landmark for its localization. The region of the AV groove just beneath the crux of the heart was carefully dissected by using microdissecting forceps (Harvard Bioscience, catalog no. 52-2086) and baby mixer hemostatic forceps (American Hospital Sup- ply, catalog no. 38025-OSS) to expose the left circumflex (LCx), AV nodal artery, and PDA. If the PDA was visible, connective and fatty tissues were removed from the surface of this artery and the dissection was extended to the origin of the AV nodal artery. Following exposure and isolation of its branches, the AV nodal artery was rubbed carefully with dry gauze to remove connective tissue around it. This allowed the anatomy of the arteries in the region of the AV groove just beneath the crux of the heart to be visualized. veins crossing over the psA and its branches were carefully isolated and deflected aside with a nonocclusive ligature.
FIG. 1.
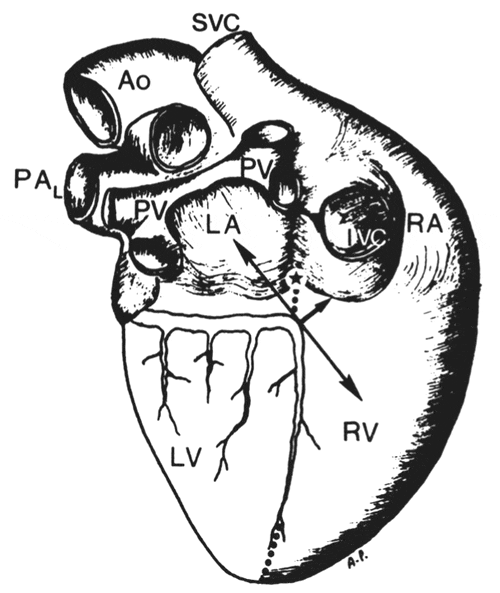 schematic drawing of canine heart [posterior view, redrawn from Sobotta and Uhlenhuth (14)] indicating-anatomical landmarks helpful for cannulation of the atrioventricular (AV) nodal artery. Crux of heart (*), which is groove between coronary sinus (CS) at this point of entrance into right atrium (RA) and inferior vena cava (IVC), is used to identify left circumflex-posterior descending artery (LCx-PDA) corner. Short thick arrow, perpendicular to an imaginary line (↔) extending from left atrium (LA) to midlateral free wall of RV, tangent to LCx-PDA corner, represents either AV nodal artery (i.e., most superior posterior septal artery) or AV nodal branch as applicable. Origin of AV nodal artery is under fatty tissue ~9-13 mm below the crux of heart. AV nodal branch originating from either PDA or AV nodal artery is always directed toward “2 o’clock” position (position of PPA is approximately at 6 o'clock) when looking at posterior surface of heart from base to apex. PDA courses along posterior interventricular septum (dotted line). Ao, aorta; LV, left ventricle; PAL, left pulmonary artery; PV, pulmonary vein; RV, right ventricle; SVC, superior vena cava.
schematic drawing of canine heart [posterior view, redrawn from Sobotta and Uhlenhuth (14)] indicating-anatomical landmarks helpful for cannulation of the atrioventricular (AV) nodal artery. Crux of heart (*), which is groove between coronary sinus (CS) at this point of entrance into right atrium (RA) and inferior vena cava (IVC), is used to identify left circumflex-posterior descending artery (LCx-PDA) corner. Short thick arrow, perpendicular to an imaginary line (↔) extending from left atrium (LA) to midlateral free wall of RV, tangent to LCx-PDA corner, represents either AV nodal artery (i.e., most superior posterior septal artery) or AV nodal branch as applicable. Origin of AV nodal artery is under fatty tissue ~9-13 mm below the crux of heart. AV nodal branch originating from either PDA or AV nodal artery is always directed toward “2 o’clock” position (position of PPA is approximately at 6 o'clock) when looking at posterior surface of heart from base to apex. PDA courses along posterior interventricular septum (dotted line). Ao, aorta; LV, left ventricle; PAL, left pulmonary artery; PV, pulmonary vein; RV, right ventricle; SVC, superior vena cava.
Cannulation technique. Following the intravenous administration of 2,000 U heparin sodium salt, the AV nodal artery was tied at its origin with a fine cotton ligature, punctured near the ligation with a 25-gauge needle, and a polyethylene cannula was inserted to a depth of 5-7 mm within the AV nodal branch. Identification of the latter is described below. The cannula was made as follows: the tip of a polyethylene tubing cannula (Intra-medic, no. 7410) was tapered to a fine point by heating and pulling. The cannula was filled with heparinized saline solution (5U/ml). The length of cannula to be positioned inside the artery was estimated before cannulation and marked with a flag (BM masking tape), which later was also used for manipulation of the cannula during insertion and for fixation. A stainless steel wire (Medwire, 316S, S10T) was inserted into the polyethylene tubing cannula to make it rigid and to facilitate its manipulation. The wire was removed after positioning of the cannula and was reinserted when repositioning was required.
The intralumen position of the cannula was confirmed by backflow of collateral blood. Poor or absent backflow suggested an improper insertion, or obstruction of the tip of the cannula. After observing the backflow, a second ligature was tied around the PSA or the "AV nodal branch", 3-5mm distal to the first ligature. Both ligatures were fixed to the masking tape flag to secure the cannula in place.
Following the approach of James et al. 7), acetylcholine chloride (0.1μg/ml, 1.0ml) followed by normal saline solution (1 ml) was administered over 3-4 s into the cannula to confirm that the cannulated blood vessel was indeed the AV nodal artery branch. Induction of transient complete heart block immediately following acetylcholine administration was considered confirmation of successful cannulation 7).
At the end of each experiment, the heart was removed with the AV cannula in place, and the LCx, all LCx branches, and the PDA were all carefully isolated, measured, and a schematic anatomical drawing was made. Blue dye solution (Siemens-Elema, mingograph ink) was injected through the cannula to visualize area perfused by the cannulated AV nodal artery. This latter postmortem gross anatomical observation was also carried out in an additional 10 hearts removed from dogs (either sex, 25-30kg) used for other physiological experiments in our laboratory.
The effect of AV nodal artery occlusion or conduction through the AV node was determined by changes in AH interval observed during right atrial pacing at fixed rate.
All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and Policy.
RESULTS
Variations of the posterior descending artery. The anatomy of the PDA was investigated in 30 canine hearts at postmortem examination, and in 20 of these animals the dissection of the PDA was also performed in the intact beating heart. In all cases, the PDA originated from the LCx. Two main anatomical variations of the PDA (variations A and B) were found as shown in Fig. 2. In variation A, a single LCx provided several marginal branches, and its distal section became the PDA. In variation B, the single LCx was divided into two main branches 10-30 mm distal to the bifurcation of the main left coronary artery. One major branch became the PDA after traveling deep inside the AV groove. The other branch ran more superficially in the AV groove and provided the marginal branches. Both variations A and B were further divided into two subtypes (I and II) according to the size of the PDA. In subtype I, the size (both diameter and length) of the PDA wis the largest of all the LCx branches. In subtype II at least one of the marginal branches of the LCx was larger than the PDA. Quantitation of the anatomical variations of the PDA is shown in Table 1. Variation A was found most frequently (26 dogs, 87%) and only 4 dogs (13%) exhibited variation B. Two-thirds of the dogs (67%) exhibited subtype II and one-third (33%) had subtype I. In some cases with subtype II, the PDA was not always readily visible. Therefore, the size of LCx branch could not be used to identify the PDA. Also, in most cases the point of origin of the PDA along the LCx was buried in the AV groove and only the distal portion of the PDA could be seen without dissection. Therefore the crux,of the heart was the most important anatomical landmark. Thus it was concluded that irrespective of its size, the branch of the LCx which travels toward the apex perpendicular to the AV groove, directly below the crux of the heart, is the PDA.
FIG. 2.
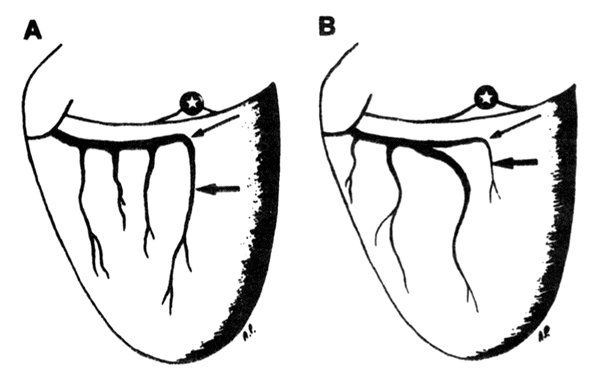 Schematic representations of variations in posterior descending artery (PDA) anatomy. Variation A (left):1 major LCx artery provides several marginal branches and becomes the PDA. Variation B (right): left circumflex (LCx) artery bifurcates into 2 large branches, 10-30 mm distal to bifurcation of left main coronary artery. One branch becomes PDA, and it courses deep inside atrioventricular (AV) groove; other branch is more superficial and it provides marginal branches. Stars designate crux of heart; longer, thinner arrows point to the LCx-PDA corner, and the thicker, shorter arrows point to the PDA.
Schematic representations of variations in posterior descending artery (PDA) anatomy. Variation A (left):1 major LCx artery provides several marginal branches and becomes the PDA. Variation B (right): left circumflex (LCx) artery bifurcates into 2 large branches, 10-30 mm distal to bifurcation of left main coronary artery. One branch becomes PDA, and it courses deep inside atrioventricular (AV) groove; other branch is more superficial and it provides marginal branches. Stars designate crux of heart; longer, thinner arrows point to the LCx-PDA corner, and the thicker, shorter arrows point to the PDA.
Variation of the AV nodal artery and its branches. After identifying the PDA, the branches originating at the "corner" where the LCx turned into PDA (LCx-PDA corner) were surveyed to identify the AV nodal artery in 30 postmortem examinations and in 20 beating hearts in vivo.
The AV nodal artery always branched off at the LCx-PDA corner except in one case. Three major anatomical variations of the AV nodal artery were found as shown in Fig. 3 and Table 1: in variation a, only one main AV nodal artery emerged from the LCx-PDA corner; it was 3-5 mm long and L mm in diameter, and it branched into several arteries. In variation b, only one AV nodal artery emerged as in variation a, but in close proximity to the point of origin it branched into several arteries. In variation c, more than one septal artery originated at the LCx-PDA corner in close proximity to each other; AV nodal artery, i.e., the most superior posterior septal artery, was relatively small in diameter and unbranched in its proximal portion. In one case with PDA variation A, subtype II, there was an anomalous variation of the AV nodal artery: the latter did not emerge from the LCx-PDA corner; however, one small artery branched off from LCx, 10 mm below the left atrial appendage and became the AV nodal artery after traveling for 25 mm deep inside the AV groove. Sixteen of 30 dogs (54%) showed variation a, 3 (10%) variation b, and 9 (30%) variation c; of the remaining two dogs, one had an anomalous variation and the other no identifiable AV nodal artery (Table 1). There was no apparent correlation between the variations of the PDA and variations of the AV nodal artery. In all cases, irrespective of the anatomical category, the branch originating from either the PDA or the AV nodal artery, attd supplyring blood to the AV node (termed here "AV nodal branch") was always directed toward the "2 o'clock" position when looking from the base to the apex at the posterior aspects of the heart (in this view, the position of PDA is approximately at 6 o'clock), and its origin was directly below the crux of the heart (Fig. 1). The point of origin of the AV nodal artery at the LCx- PDA corner was 9-13 mm below the crux of the heart. In 25 of 30 dogs (83%), postmortem examination (Fig. 4B) and dye injection into the branch directed at the 2 o'clock position resulted in staining of the AV nodal region. only one dog did not exhibit such a branch directed in the 2 o'clock position and dye injection into all branches arising at the LCx-PDA corner failed to stain the AV nodal region. The remaining four dogs exhibited a branch oriented in the 2 o'clock position, however attempts at dye injection were unsuccessful, probably due to thrombotic occlusion.
TABLE 1. Frequency of anatomical variations of the posterior descending and AV nodal coronary arteries
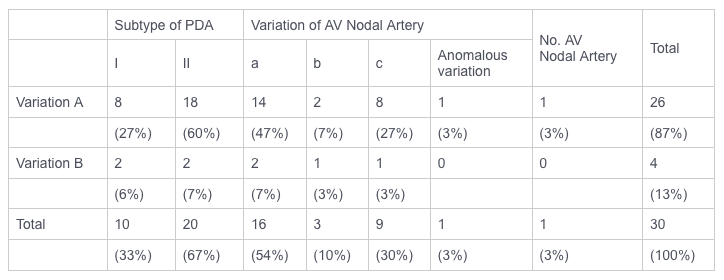 AV, atrioventricular; PDA, postterior descending artery.
AV, atrioventricular; PDA, postterior descending artery.
FIG. 3.
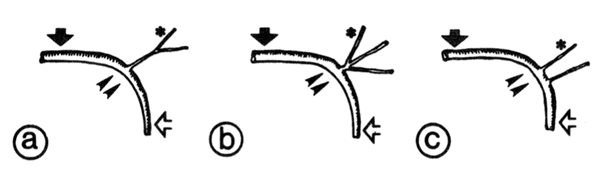 Schematic representation of variations in anatomy of atrioventricular (AV) nodal artery and its branches. Variation a (left): only 1 major AV nodal artery emerges from left circumflex artery-posterior descending artery (LCx-PDA) corner (two arrowheads) with a common portion 3-5 mm long and 1 mm in diam. Varriation b (middle): only 1 AV nodal artery emerges as in variation a,but in close proximity to its point of origin it branches into several arteries. Variation c (right): more than t posterior septal artery is found at LCx-PDA corner. Most commonly found AV nodal branch in each anatomical variation is labeled with asterisk; filled large arrowheads point to the LCx artery and open arrows to PDA.
Schematic representation of variations in anatomy of atrioventricular (AV) nodal artery and its branches. Variation a (left): only 1 major AV nodal artery emerges from left circumflex artery-posterior descending artery (LCx-PDA) corner (two arrowheads) with a common portion 3-5 mm long and 1 mm in diam. Varriation b (middle): only 1 AV nodal artery emerges as in variation a,but in close proximity to its point of origin it branches into several arteries. Variation c (right): more than t posterior septal artery is found at LCx-PDA corner. Most commonly found AV nodal branch in each anatomical variation is labeled with asterisk; filled large arrowheads point to the LCx artery and open arrows to PDA.
Incidence of successful cannulation. Cannulation of the AV nodal branch in the beating heart was attempted in 20 dogs, divided into two groups. In the initial five dogs (group I), which were used to establish the methodology, only two showed successful cannulation, which was defined as successful identification, isolation, and cannulation of AV nodal artery, physiological confirmation by acetylcholine injection (transient AV conduction block) (7) and anatomical confirmation (staining of the AV nodal region by dye). In the remaining three dogs in group I cannulation failed due to technical reasons (i.e., failure to identify correctly the proper branch in 1 case and incomplete dissection in 2 cases). Adhering to the anatomical landmarks described above and identified during the initial 5 dog cannulation trials, 15 dogs (group II) were tested to verify the usefulness of our methods. The results of attempted cannulation in group II are shown in Fig. 4A. ln 3 of the 15 dogs (20%) in group II, the AV nodal artery was anatomically unsuitable for cannulation: in one case, no AV nodal artery could be found; in another, the AV nodal artery was too small for cannulation; and in the third dog, an anomalous varia- tion of the AV nodal artery was present. In 12 of these 15 dogs (80%), the AV nodal artery was anatomically suitable for cannulation. Successful cannulation, con- firmed by the above definition, was achieved in 10 of these 12 dogs (83%). A representative example of successful cannulation is shown in Fig. 5. In the remaining two dogs (17%), acetylcholine induced ventricular fibrillation in one and AH interval prolongation, but not AV block in the other. These two dogs also failed to show anatomical confirmation (i.e., staining of the AV node) by dye injection.
FIG. 4.
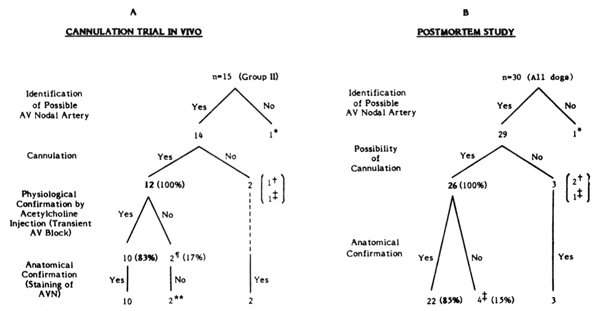 Results of in vivo (A) and postmortem (B) cannulation attempts of atrioventricular (AV) nodal artery.
Results of in vivo (A) and postmortem (B) cannulation attempts of atrioventricular (AV) nodal artery.* No AV nodal artery could be found. † AV nodal branch too small for cannulation. ‡ Anomalous variation of AV nodal artery. ¶ Acetylcholine injection resulted in AH interval prolongation but no AV block in one case and immediate occurrence of ventricular fibrillation in other. ** Failure to inject dye into the AV nodal branch, probably due to thrombotic occlusion.
FIG. 5.
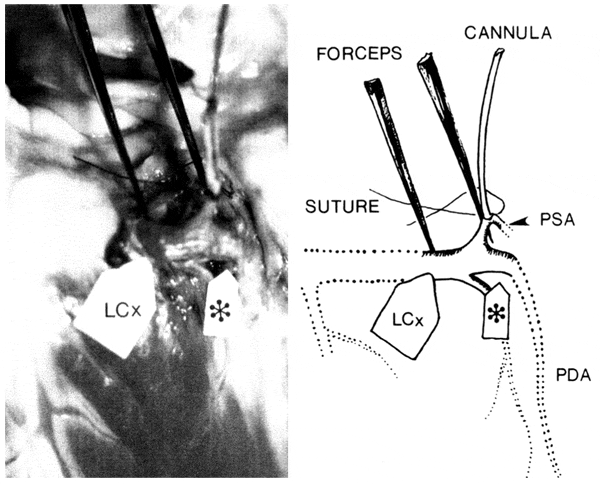 Cannulated atrioventricular (AV) nodal artery in a canine heart (posterior view) in situ (left) with schematic drawing (right). Left circumflex artery-posterior descending artery (LCx-PDA) corner (*) and AV nodal artery are isolated and a cannula is placed in latter.Since LCx-PDA corner was retracted with forceps, AV nodal artery appears to have a different orientation from its natural "2 o'clock" position. According to our classification, this shows variation A, subtype I (PDA)-variation a (AV nodal artery). Dotted lines indicate non-isolated vessels. LCx, left circumflex coronary artery; PDA, posterior descending artery; PSA, most superior posterior septal artery equals AV nodal artery.
Cannulated atrioventricular (AV) nodal artery in a canine heart (posterior view) in situ (left) with schematic drawing (right). Left circumflex artery-posterior descending artery (LCx-PDA) corner (*) and AV nodal artery are isolated and a cannula is placed in latter.Since LCx-PDA corner was retracted with forceps, AV nodal artery appears to have a different orientation from its natural "2 o'clock" position. According to our classification, this shows variation A, subtype I (PDA)-variation a (AV nodal artery). Dotted lines indicate non-isolated vessels. LCx, left circumflex coronary artery; PDA, posterior descending artery; PSA, most superior posterior septal artery equals AV nodal artery.
The suitability of the AV nodal artery for cannulation was assessed during postmortem examination (Fig. 4B). Twenty-six of 30 dogs (87%) had suitable anatomy for AV nodal artery cannulation, The remaining four dogs (13%) showed unsuitable anatomy of AV nodal artery cannulation due to the reasons mentioned above. Twenty-two of the 26 dogs (85%) with suitable anatomy showed postmortem staining of the AV node. The remaining four dogs (15%) failed to show staining of the AV node. The reasons for this were not clear, but one probable explanation was thrombotic occlusion of the AV nodal artery. Thus postmortem staining of the AV node showed an 85% possibility of successful cannulation if the AV nodal branch was correctly identified.
In addition, there was no apparent correlation between the variation of PDA and AV nodal artery, and the anatomical suitability of the latter for cannulation. In both variations b and c, a cannula was successfully placed in the AV nodal branch
Longevity of the preparation. Interruption of blood flow through the AV nodal artery resulted in deterioration of AV nodal performance judged by progressive prolongation of AH interval during right atrial pacing. In these studies no change in AH interval was noticed during the first 60 min of cannulation.
DISCUSSION
Cannulation of the canine AV nodal artery for selective perfusion of the AV node is a useful method for studying the electrophysiological effects of various pharmacological agents, metabolites, and alterations in blood flow 1) 2) 3) 4) 7) 8) 12) 15) 16) 17) 18). However, several difficulties could be encountered when following the original method of Nadeau and Amir-Jahed 11). Foremost, information on variations in the anatomy of the AV nodal artery were lacking and thus identification of the coronary artery that supplies blood to the AV node is difficult. In the present study, the AV nodal artery, which is the most superior posterior septal artery, originated at the LCx- PDA corner in all cases except one, in agreement with previous findings 5) 7) 9). Three major anatomical variations in the origin of the AV nodal artery were observed. According to the description of Nadeau and Amir-Jahed 11), this artery, which measured 3-5 mm in length, originated from LCx, and in less than half of the cases it had a major branch to the right ventricle. This anatomy matches variation a of our classification. However, even in this variation, several small branches of the AV nodal artery were always found with more extensive, careful dissection. Lumb et al. 9) also suggested that the AV nodal artery branches into several small arteries that supply blood not only to the AV node but also to the superior aspect of the right ventricular wall and the left and right atrium. Therefore, in these previous studies cannulation of the AV nodal branch was in fact cannulation of the AV nodal artery and the cannula was inserted only a few millimeters deep to avoid placing its tip distal to the origin of the AV nodal branch and into the "wrong" branch. However, using the presently de- scribed anatomical landmarks and orientation, a cannula can be inserted directly into the AV nodal branch. This deeper insertion is also helpful to secure the cannula. Moreover, Nadeau and Amir-Jahed 11) did not report the several anatomical variations that have been classified in the present study including variation b, although they did describe variation c, which they reported to be unsuitable for cannulation due to the small size of the coronary artery designated for cannulation. However, 10% of cases in the present study showed variation b; and in the larger dogs with variation c, at least two vessels which originated form the LCx in each dog were large enough to permit successful cannulation.
Another difficulty encountered in following Nadeau and Amir-Jahed's technique was localization of the region where the AV nodal artery originates from the LCx- PDA corner. This area is covered by fatty tissue and is not visible without dissection. The LCx is also covered by fatty tissue; hence it is important to identify the PDA first, before isolating the AV nodal artery. However, as described above, the PDA was not always large and readily visible. The crux of the heart, below which the LCx curves and turns into the PDA as suggested by previous investigators 6) 7) 9) must be emphasized as an important anatomical landmark helpful in identifying the PDA. In large dogs, the origin of the AV nodal artery at the LCx-PDA corner is found under fatty tissue ~9- 13 mm posterior to the crux of the heart, irrespective of the PDA anatomical variation. In this region, coronary veins might cross over the AV nodal artery, and therefore, they have to be carefully isolated and deflected to enable its exposure. After finding the origin of the AV nodal artery, all of its branches within its initial segment (5-8 mm) should also be exposed. At this stage it is easy to define the AV nodal branch directed at the 2 o'clock position.
The present study also confirms previous reports 5) 6) 10) 13) indicating that the PDA continues from the LCx in all cases in the dog and never from the right coronary artery. Two variations of the PDA (A and B) were found. Approximately 90% of the dogs showed variation A, and 10% showed variation B. In variation B, the LCx usually ran deep inside the AV groove and the AV nodal artery emerged from the LCx-PDA corner which was deeper than in variation A. Therefore, when encountering variation B more extensive dissection and time is necessary for exposing the AV nodal artery, therefore the investigator may be advised to abort the AV nodal cannulation in these preparations.
The size of the dog is another critical factor for successful cannulation of the AV nodal artery. In the present study, relatively large adult mongrel dogs (25-30 kg) were used compared with previous studies (10-24 kg); this was probably helpful in the dissection of the various arteries.
Postmortem staining of the AV node showed an 85% possibility of successful cannulation if the AV nodal branch was correctly identified and the vessel rvas not too small for cannulation. The frequency of successful cannulation in the 15 group II dogs in this study, following studies in the initial b cases (group I) to establish methodology, approached this percentage. Therefore, the present cannulation technique is readily learned. It must be emphasized, however, that the success of this technique also depends on the quality of the cannula. In preparing,a polyethylene tubing cannula, the tip should be tapered to a fine point, which facilitates easy insertion of the cannula into the vessel via the puncture hole made by a 25-gauge needle. Postmortem study showed that 15% of dogs were anatomically unsuitable for AV nodal artery cannulation. Therefore, to avoid animal wastage, investigators should be prepared to use these animals for alternative protocols.
In the present study, anatomical examination was done to help define readily identifiable and reliable anatomical landmarks, which could be used to facilitate successful AV nodal artery cannulation for physiological studies. Although we have described an anatomical classification based on our observations in 30 dogs, a more definitive anatomical classification by anatomists may be war- ranted when larger numbers of dogs have been studied. Nonetheless, for the purpose of investigating the physiological effects of selectively altering the composition of blood perfusing the AV node or its pattern of flow, pharmacological agents on AV node using AV nodal cannulation technique, it appears that the present classification system should be very useful.
Two important aspects of blood supply to the AV nodal region should be remembered. 1) Cannulation of the AV nodal artery does not interfere with the blood supply to the proximal part of the AV node by the anterior septal artery. 2) Since collateral blood supply to the AV node region is limited, prolonged periods of cannulation of AV nodal artery require the delivery of blood or oxygenated physiological solution to the AV node using, for example, a carotid-to-AV nodal artery shunt.
In conclusion, recognition of the anatomical variations of the canine PDA and AV nodal artery can facilitate cannulation of the coronary AV nodal branch with a high success rate using the techniques described.
We are grateful to carl Hurt and Wayne Klim for technical assistance in the performance of experiments and to Rose Marie wells for preparation of the manuscript.
This study was supported in part by Clinical Investigator Award KO8 HL-01312 from the National Heart, Lung, and Blood Institute, to E. L. Michelson.
Received 12 January 1987; accepted in final form 19 May 1987.
REFERENCES
- Belardinelli, L., E. C. Mattos, AND R. M. Berne. Evidence for adenosine mediation of atrioventricular block in the ischemic canine myocardium. J. CIin. Invest.68: 195-205, 1991.
- Epstein, A. E., F. Urthaler, and T. N. James. No electrical instability after intracoronary streptokinase administered into sinos node or AV node of dogs. Am. Heart J. 107: 902-905, 1984.
- Gauthier, P., and R. A. Nadeau. Effects of nicotine injected into the atrioventricular node artery of the dog. J. Pharmacol, Exp. Ther. 169: 298-307, 1969.
- Iijima, T., S. Motomura, N. Taira, and K. Hashimoto. Selective suppression of neural excitation by tetrodotoxin injected into the canine atrioventricular node artery. J. Pharmacol. Exp. Ther. 189: 638-645, 1974.
- James, T. N. Anatomy of the Coronary Arteries. New York: Hoeber, 1961, p. 162-172.
- James, T. N. Anatomy of the AV node of the dog. Anat. Rec. 148: 15-18, 1964.
- James, T. N., E. S. Bear, R. J. Frink, K. F. Lang, and J. C. Tomlinson. Selective stimulation, suppression, or blockade of the atrioventricular node and His bundle. J. Lab. Clin. Med. 76: 240-256, 1970.
- James, T. N., E. S. Bear, R. J. Frink, and F. Urthaler. Pharmacologic producion of atrioventricular block with and without initial bundle banch block. J. Pharmacol. Exp. Ther. 179: 338-346, 1971.
- Lumb, G., R. S. Shacklett, and W. A. Dawkins. The cardiac conduction tissue and its blood supply in the dog. Am. J. Pathol. 35: 467-487, 1959.
- Moore, R. A. The coronary arteries of the dog. Am. Heart J. 5: 743-749, 1930.
- Nadeau, R. A., and A. K. Amir-Jahed. Selective perfusion of the AV node of the dog by cannulation of the posterior septal artery. rev. Can. Biol. 24: 291-297, 1965.
- Nadeau, R. A., and A. K. Amir-Jahed, P. Gauthier, and G. A. Porlier. Effects of cardiac glycosides injected into the atrioventricular node artery of the dog. Can. J. Physiol. Pharmacol. 49: 113-126, 1971.
- Pianetto, M. B. The coronary arteries of the dog. Am. Heart J. 18: 403-410, 1939.
- Sobotta, M. J., and E. Uhlenhuth. Atlas of Desciptive Human Anatomy. New York: Hafner, 1957, p. 199.
- Urthaler, F., and T. N. James. Effect of adenosine and ATP on AV conduction and on AV junctional rhythm. J. Lab. Clin. Med. 79: 96-105, 1972.
- Urthaler, F., andT. N. James. Effect of tetrodotoxin on A-V conduction and A-V junctional rhythm. Am. J. Physiol. 2224:1155-1161, 1973.
- Yano, K., M. Hayano, F.Kiya, M. Fukatani, Y. Matsumoto, T. Mitsuoka, and K. Hashiba. Effect of verapamil directly administered into A-V node artery on A-V junctional automaticity and A-V conduction in dogs. J. Cardiovasc. Pharmacol, 7: 1027-1033, 1985.
- Zipes, D.P., and J. C. Fischer. Effects of agents which inhibit the slow channel on sinus node automaticity and atrioventricular conduction in the dog. Circ. Res.34: 184-192, 1974.



